Ozone Layer Information Platform
OZONE LAYER
What is ozone layer?
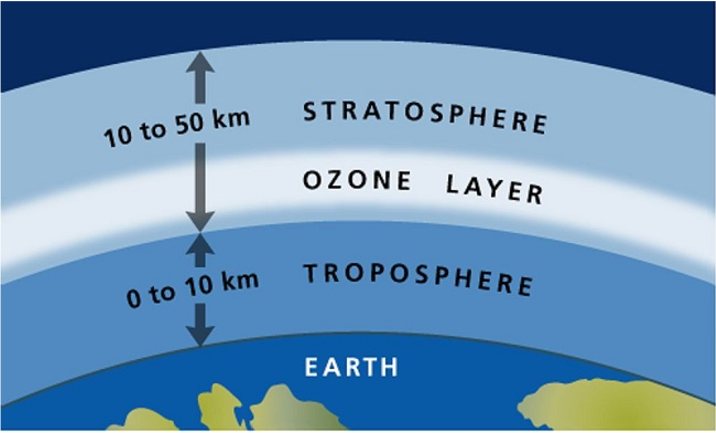
Ozone is a gas that is naturally formed by the combination of three oxygen atoms in the atmosphere. It is chemically denoted as O3. 90% of ozone in the atmosphere is found in the upper part of the stratosphere between 15-50 km from the ground surface. The gas layer formed by the total ozone in this range is called the Ozone Layer. The ozone layer prevents harmful UV radiation such as UV-B and UV-C from the Sun from reaching the Earth's surface. In this respect, the Ozone Layer is very important for the sustainability of life on Earth and human health. The remaining 10% ozone amount in the atmosphere is in the troposphere layer. The ozone here is formed by the accumulation of harmful chemicals released into the air anthropogenically, reacting and accumulating.
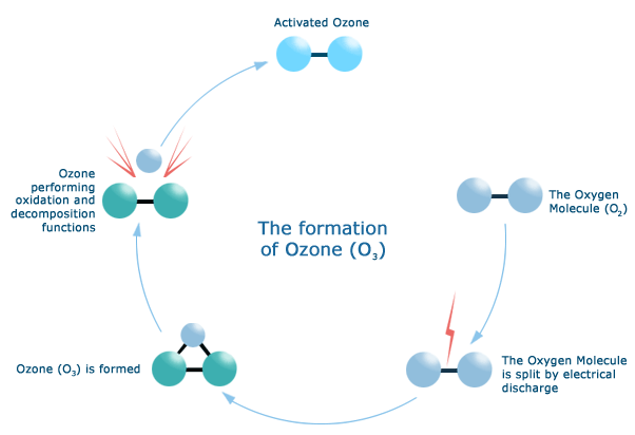
How is the Ozone Layer formed?
Free oxygen, which was formed with the photosynthesis of living things, began to accumulate in the stratosphere layer. The sun's UV radiation hit the oxygen molecules in this layer, causing these molecules to split into two oxygen atoms. Free oxygen atoms react with other oxygen molecules to form the ozone molecule. So, these reactions increase the amount of ozone in the stratosphere. In this way, the ozone layer was formed.
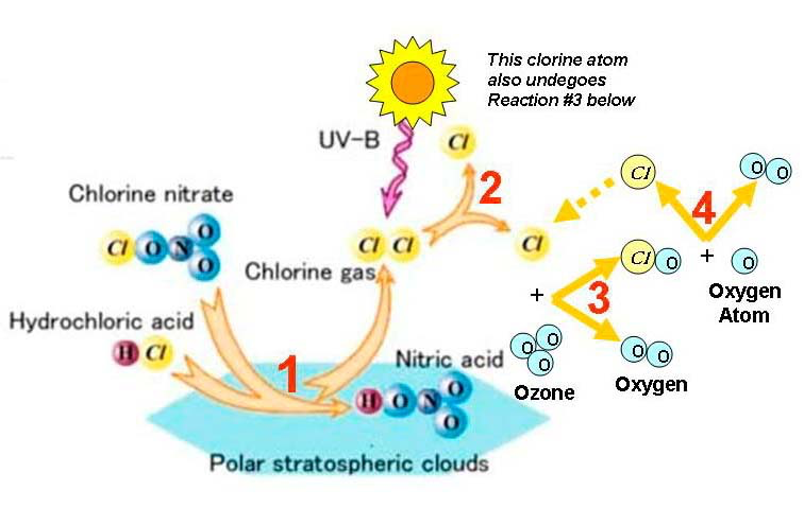
What is Ozone Hole? How is the Ozone Layer depleted?
The depletion of the Ozone Layer means the great and rapid decrease in the ozone molecules. Some chemical substances cause the depletion of the Ozone Layer. The main ones are chlorofluorocarbons (CFCs) containing hydrogen, nitrogen, chlorine and bromine, hydrochlorofluorocarbons (HCFCs), halons, methichlorform, methylbromuracid.
After their discovery in the 1930s, CFCs began to be used in industries as cooling agents, blowing agents, cleaning agents and foams since they were less toxic, chemically stable and cheap. Chlorine atoms emitted into the atmosphere as a result of the heavy use of CFCs can travel up to the stratosphere without any deterioration. Here, they disintegrate with the UV energy received from the sun and reveal chlorine atoms. Ozone molecules that react with free chlorine atoms form chlorine monoxide molecules. The chlorine monoxide molecules reacts with the free oxygen atoms in the ozone layer and creates chlorine atoms and oxygen molecules that will destroy more ozone molecules. As a result of the repetition of this reaction over and over, it has been understood that one chlorine atom can destroy approximately 100,000 ozone molecules.
The thinning of the ozone layer is mostly observed in the South Pole. The reason for this is that chemical reactions at the poles are more effective. High levels of chlorine and bromine ions due to low temperature damage to the ozone layer more than other parts of the world.
In the mid-1970s, scientists realized that the ozone layer was threatened by the accumulation of gases containing halogens (chlorine and bromine, or CFCs) in the atmosphere. Then, in the mid-1980s, scientists discovered a “hole” in the ozone layer above Antarctica – the region of Earth’s atmosphere with severe depletion. After it was noticed, the use of CFCs was banned all over the world with the Montreal Protocol in 1987. In recent studies, it has been observed that especially with the prohibition of the use of CFCs, the depletion of the ozone layer has stopped, and the layer has been self-repairing and thickening.
Why is the Ozone Layer important?
Harmful sunlight (UV-B and UV-C) reaching the earth as a result of ozone depletion is a detrimental effect on all living things, including humans, from the simplest single-celled plants to insects, fish, birds and mammals. With the thinning of the Ozone Layer, there will be an increase in cataract and immune system diseases, especially skin cancer. For this reason, the Ozone Layer is one of the most important elements that help maintain vitality by protecting ecological balance.
IT IS POSSIBLE TO PROTECT OZONE LAYER!
The success of the Montreal Protocol means that 99 percent of ozone depleting substances (ODSs) are now controlled. However, the protection of the ozone layer depends on our common position. By applying small steps in our daily life, we can contribute to the protection of the ozone layer.
 Never use sprays containing CFC and HCFC.
Never use sprays containing CFC and HCFC.
 Purchase ozone friendly products that do not contain chemicals that damage the ozone layer. Use your strength as a consumer.
Purchase ozone friendly products that do not contain chemicals that damage the ozone layer. Use your strength as a consumer.
 By using refrigerators, air conditioners and other equipment responsibly, you would assist in protecting the ozone layer and climate too. Regularly service older equipment (not CFC free) as their malfunctions cause CFC to escape into the atmosphere. When your equipment needs to be serviced, ask for trained, qualified and certified experts to ensure the equipment is properly repaired and carrier gases are recycled.
By using refrigerators, air conditioners and other equipment responsibly, you would assist in protecting the ozone layer and climate too. Regularly service older equipment (not CFC free) as their malfunctions cause CFC to escape into the atmosphere. When your equipment needs to be serviced, ask for trained, qualified and certified experts to ensure the equipment is properly repaired and carrier gases are recycled.
 Dispose of appliances and equipment with refrigerants responsibly. When you buy a new appliance such as a refrigerator, look for products that are labeled ‘ozone friendly’ or ‘HCFC free’ or have an energy efficiency label.
Dispose of appliances and equipment with refrigerants responsibly. When you buy a new appliance such as a refrigerator, look for products that are labeled ‘ozone friendly’ or ‘HCFC free’ or have an energy efficiency label.
 Set the thermostat of your refrigerator and freezer at the right temperature by avoiding too low temperatures. A warmer setting of your air conditioner thermostat will also save energy. Switch equipment off when not in use as even a standby mode consumes energy.
Set the thermostat of your refrigerator and freezer at the right temperature by avoiding too low temperatures. A warmer setting of your air conditioner thermostat will also save energy. Switch equipment off when not in use as even a standby mode consumes energy.
 Cooling systems depend on good airflow through their heat exchange panels (evaporators and condensers). Mount your refrigerator so that air can easily circulate at the back and do not put the refrigerator freezer next to an oven or dishwasher.
Cooling systems depend on good airflow through their heat exchange panels (evaporators and condensers). Mount your refrigerator so that air can easily circulate at the back and do not put the refrigerator freezer next to an oven or dishwasher.
 Clean the refrigerator regularly at the back where the condenser is located and de-ice the freezer regularly.
Clean the refrigerator regularly at the back where the condenser is located and de-ice the freezer regularly.
 Dispose your old refrigerator by taking it to a qualified and certified expert, as refrigerators and freezers contain refrigerants that must be removed and recovered before other parts are recycled.
Dispose your old refrigerator by taking it to a qualified and certified expert, as refrigerators and freezers contain refrigerants that must be removed and recovered before other parts are recycled.
 Keep rooms cool at night with ventilation, without air-conditioning if possible. But if you need extra cooling, consider turning the temperature up - when you're sleeping, you probably don't need the air conditioner set as cold as you might think, and also remember that a higher setting of your air conditioner’s thermostat saves a lot of energy.
Keep rooms cool at night with ventilation, without air-conditioning if possible. But if you need extra cooling, consider turning the temperature up - when you're sleeping, you probably don't need the air conditioner set as cold as you might think, and also remember that a higher setting of your air conditioner’s thermostat saves a lot of energy.
 Automobile air conditioners may contain substances that damage the ozone layer as refrigerant. Nowadays, there are models that do not contain these items, prefer them when buying a new car.
Automobile air conditioners may contain substances that damage the ozone layer as refrigerant. Nowadays, there are models that do not contain these items, prefer them when buying a new car.
 Turn on the air conditioning of your car after driving for a few minutes with the windows open and park your car in the shade.
Turn on the air conditioning of your car after driving for a few minutes with the windows open and park your car in the shade.
 Remember that insulation materials and packaging foams will also contain chemicals that damage the ozone layer, so do not consume these products unnecessarily or consume effectively.
Remember that insulation materials and packaging foams will also contain chemicals that damage the ozone layer, so do not consume these products unnecessarily or consume effectively.
 Learn more about chemicals that damage the ozone layer and products that contain them, and inform the people around you.
Learn more about chemicals that damage the ozone layer and products that contain them, and inform the people around you.
HOW SHOULD WE PROTECT OURSELVES?
Avoiding exessive sun exposure.
 Wearing Sunglasses
Wearing Sunglasses
Sunglasses are so much more than a fashion accessory or just for reducing the sun’s glare. The lenses that filter out ultraviolet (UV) rays help protect the eyelid, cornea, lens and retina. When choosing sunglasses, they should have UV protective glasses.
 Using Sunscreen.
Using Sunscreen.
Too much sun causes damage to skin and eyes, but our skin also produces vitamin D when it is exposed to sunlight. When outdoors in the sun, use a sunscreen that provides good UV protection with a sun protection factor (SPF) rating of 30 or higher. Put on sunscreen 15 to 20 minutes before going out in the sun. Remember to reapply regularly if continuously exposed to the sun, especially if you are swimming or sweating a lot – even if the sunscreen states it’s waterproof! Try to limit the time spent in direct sun try to avoid sunburn and wear a wide-brimmed hat and protective clothing. And if you are a parent, make sure you protect your child's skin. Overexposure to sunlight before age 18 is most damaging to the skin so start practicing safe sun habits and awareness from an early age.
 Checking UV index
Checking UV index
Pay attention to the ultraviolet (UV) index, an international standard measurement of the strength of sunburn-causing UV radiation at a particular place and time, which gives you an idea of how strong the UV light is in your area on any given day, on a scale from 1 to 11+..
A higher number means greater risk of exposure to UV rays and a higher chance of sunburn and skin damage that could ultimately lead to skin cancer. The UV Index is part of many weather forecasts.
Awareness of the UV index and the impact of exposure to UV radiation on our skin is key. Some people get a sunburn faster than others because of their colouring so be aware of what is ‘normal’ for you and how quickly your skin goes red. If you have blonde or red hair, light-coloured skin, and light-coloured eyes, you'll tend to get a sunburn more quickly than someone with dark eyes and skin. That's because you have less melanin, a chemical in the skin that protects it from sun damage by reflecting and absorbing UV rays.
 Using your shadow to measure UV levels
Using your shadow to measure UV levels
An easy way to tell how much UV exposure you are getting is to look for your shadow. If your shadow is taller than you are (in the early morning and late afternoon), your UV exposure is likely to be lower. If your shadow is shorter than you are (around midday), you are being exposed to higher levels of UV radiation. Seek shade and protect your skin and eyes. Other factors to consider when assessing UV exposure include the season of the year: UV rays are stronger during spring and summer months, depending in your geographical location. This is less of a factor near the equator. UV exposure goes down as you get further from the equator but will increase at altitude because more UV rays reach the ground at higher elevations. It is also important to be mindful that UV rays can bounce off surfaces like water, sand, or snow. Lastly, UV rays can also reach below the water’s surface, so your skin can still get sun damaged even if under water and feeling cool.



